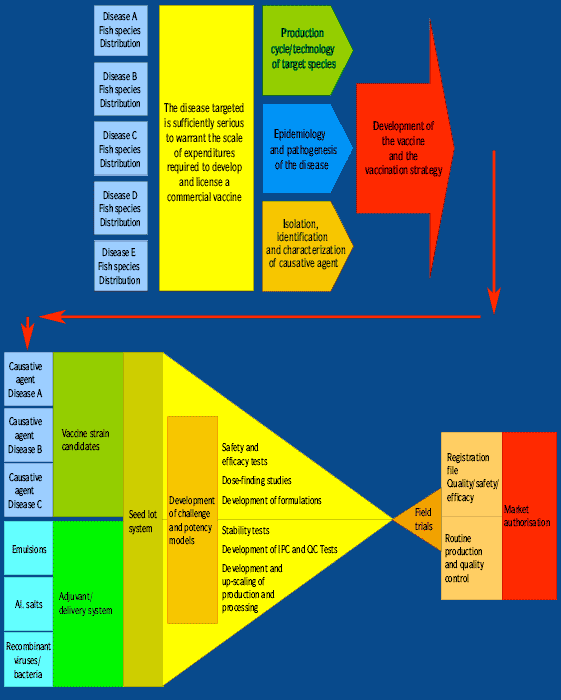
While the development of the first vaccines have been a rather empirical process, current efforts to develop vaccines have turned largely to biotechnology, depending upon detailed knowledge of aspects of the epidemiology of the disease, aspects of the biochemistry of the pathogen and the host, the identification of the key virulence factors and the production of protective antigens, focussing on the induction of an optimal and long lasting immune response.
 |
Recognition and epidemiology of the disease In-depth knowledge of the epidemiological factors of a particular disease, including its occurrence and spread, target species, biology of the host and pathogen, pathogenicity and pathogenesis, and prevalence and incidence in a population is important to understanding the disease and to identify the targets for a protective immune response. The current host and geographic range of the disease, as well as its potential for further dissemination, needs to be evaluated. |
 |
 |
Isolation of pathogen and characterisation of disease Isolation of presumptive pathogens and a full description of the disease symptoms is critical. It is not unusual for a particular disease of fish to have more than one etiological agent or to be exacerbated by another disease. Furthermore, it is important to work with virulent strains recently isolated from moribund host species showing typical clinical signs of the disease. |
 |
 |
Identification of species, strains and serotypes of the causative agent Absolute identification of the causative pathogen using the most up-to-date techniques (including LPSstained gels, gel electrophoresis and slide agglutination tests). Species of bacterial and viral pathogens often show variation in serotype groupings. The possible geographic and host specific differences of a given pathogen must be considered. |
 |
 |
Key virulence factors and protective antigens Identification of key virulence factors and protective antigens, followed by the development of an appropriate challenge model, which should simulate the natural disease condition as much as possible and must consistently provide reproducible and statistically significant data, must be developed. |
 |
 |
Experimental production Lab scale production of protective antigens and formulation of an experimental vaccine should be developed. Determination of optimal proportions of antigens: Due to interaction among antigens, it is important to determine the optimal proportions of multiple antigens to ensure the best and most complete protective immune response. |
 |
 |
Production/downstream processing/quality control/GMP Both production and downstream processing can be critical issues. Certain technical processes are difficult, costly or even impossible to scale up from the laboratory bench to the production volumes needed. Packaging/delivery: The practicality and convenience of packaging and services are further value added product characteristics. |
 |
Further Information
To view the full leaflet, click here (PDF)
December 2005

