One option is the production of sterile stocks by triploid induction. Triploidy is not a new concept, originally tested in the early 1990’s as a means to prevent maturation. Unfortunately, poor performance, higher mortalities and deformities led to the industry abandoning triploidy in favour of photoperiod control of maturation prior to harvest. However, although photoperiod does reduce maturation in culture, farmed stocks remain reproductively competent and the threat of genetic pollution following escapees persists. Triploidy is therefore at present the only non-GMO method that can produce sterile fish and the industry is now keen to reexplore this avenue.
It is for this reason that a transnational collaborative project between key R&D (Stirling University, Institute of Marine Research: Bergen and Wageningen University) and industry partners (Landcatch Natural Selection, Aquagen, Marine Harvest, Centre of Aquaculture Competence and Salmo) in Scotland, Norway, Netherlands and France was established in June 2008 as part of the EC funded Capacities Program “Research for the benefit of SMEs” to explore the feasibility of commercial triploid Atlantic salmon production. The project entitled SALMOTRIP is being lead and co-ordinated by Dr Hervé Migaud and Dr John Taylor at the Institute of Aquaculture.

The project focuses on six key areas regarding triploid salmon production: optimise induction protocols; identify physiological requirements and sensitivities; develop out-of-season smolt regimes; explore selective breeding programmes specific to triploid; commercial field trials; and examine public perception, marketing and consumer acceptance. Data generated will also aid legislative decision making regarding future aquaculture policies.
Partners have established a series of experimental and full-scale field trials at all levels of production. The first batches of triploid salmon were produced in early 2008, with second year classes produced for 2009 (Figure 1). During hatchery rearing survival to hatch did not differ between ploidy but was significantly affected by family. Survival was found to strongly correlate with gamete quality, particularly in triploids. As with survival, growth appears to be strongly affected by family, and through correct selection, triploids were found to outperform their diploid siblings with minimal deformity rates (Figure 2).
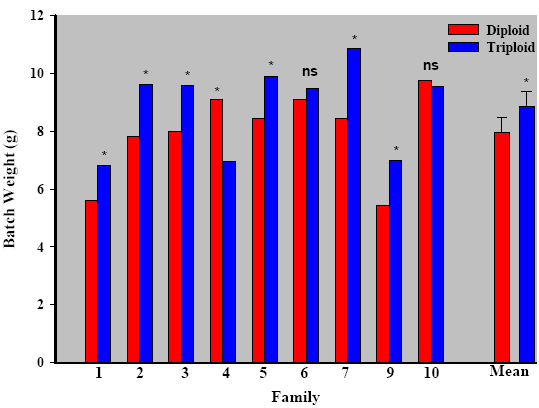
These observations are a major advancement over earlier studies and suggest that with correct broodstock selection, triploid salmon can perform as well and even better than their diploid siblings. It will also be important to determine if families that perform well in freshwater stages perform as well during saltwater grow out. Furthermore, we are evaluating the best families on traits of interest (growth, flesh quality, disease resistance etc.) observed in field trials by genotyping. Together these findings open up exciting new avenues for research into triploid-specific selection programmes and family-ploidy interactions.
Seasonal issues
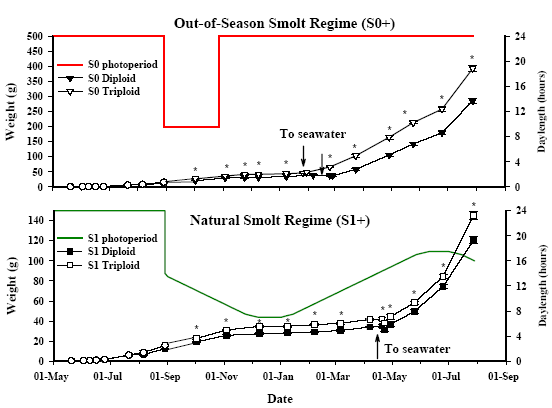
One of the fundamental successes of the salmon industry has been the ability to produce out-of-season (S0+) smolts to ensure year round availability. However, to date, studies in triploid Atlantic salmon have only focused on natural (S1) smolt production. It is thus essential that knowledge of how to produce S0+ triploid be available if farmers are to adopt this strategy. In this respect we have recently demonstrated for the first time that S0+ triploid smolts can be successfully produced, and that they show superior performance, attaining a larger size and allowing earlier seawater stocking (Figure 3).
We are continuing to investigate important criteria to consider when manipulating smoltification time in triploid salmon, particularly size-growth-lipid thresholds, and osmoregulatory physiology: in addition to refining the photo-thermal regimes themselves with regards to timing and duration.
Currently, out-of-season triploid Atlantic salmon have been transferred to sea in the Institute of Aquaculture marine facilities (Machrihanish Environmental Research Laboratories) where they will be monitored for long-term seawater performance. Results so far are very encouraging with triploid postsmolts continuing to out grow their diploid siblings under both accelerated and natural regimes (Figure 4). However, long-term sea monitoring will continue to determine whether previously reported deformities and poorer growth are a function of the change in environment or are carried genetically. Colleagues in Norway are currently exploring the effects of sub-optimal environmental rearing conditions and dietary requirements on deformity prevalence and seawater performance in relation to family-ploidy interactions.

Findings to date would indicate significant ploidy differences, particularly with regards to dietary nutrient availability. Overall the project is at a very exciting stage with knowledge growing rapidly in terms of transfer of methodologies, knowledge on triploid biology and requirements and marketing strategies to SMEs.
However, this is only the tip of the iceberg and considerable research is required in the fields of nutrition, immune competence, vaccine development, and family selection criteria to name but a few. Only when this knowledge is available can the potential for triploidy be truly realised within the salmon farming industry.
March 2010

