Although treatment and prevention are key strategies for limiting the economic and biological impact of disease, the options available in aquaculture for managing sick animals have declined due to tighter governmental regulations.
Animal health and food safety are crucial in the aquaculture industry because seafood products must be perceived by consumers as healthy and wholesome. Antibiotics that have been approved by the US Food and Drug Administration (FDA) are necessary for animal welfare and the treatment of disease caused by bacteria and some protozoa in aquatic and terrestrial animals. Safe, effective, FDA-approved antibiotics will continue to be required if the agriculture industry is to produce high-quality protein from animals destined for human consumption, but must be used judiciously to minimise the risk of antibiotic resistance and to help ensure products are safe for human consumption.
In aquaculture, antibiotics are a first line of defense for combating bacterial infections.
Aquaflor has the potential to help control mortality in many different fish species that become infected by florfenicol-sensitive bacterial pathogens. Florfenicol, the active ingredient in Aquaflor, is a broad-spectrum antimicrobial compound with both bacteriostatic and bactericidal properties, and it has activity against gram-positive and gram-negative bacteria. Aquaflor has been approved in more than 20 countries for controlling mortality due to various bacterial pathogens in a variety of fish species. In the US, Aquaflor is currently approved for controlling mortality in catfish with enteric septicemia and in freshwater-reared salmonids with coldwater disease and furunculosis; it is conditionally approved for control of mortality in catfish due to columnaris.
Currently, however, there are no antibiotics approved in the US to help control mortality from Streptococcus iniae in hybrid striped bass (HSB), which has quickly become a major aquaculture pathogen that can directly affect the successful culture of many warmwater fish species worldwide, including HSB in the US.
A single laboratory trial has demonstrated that feed medicated with Aquaflor can reduce mortality in sunshine bass experimentally infected with S. iniae. In an effort to gather information about the potential expanded use of Aquaflor in the US and abroad, considerable field data has been generated under Investigational New Animal Drug studies and supports the claim that Aquaflor can control mortality in HSB during natural S. iniae infections. This paper will provide a brief overview of S. iniae as an aquaculture pathogen in HSB, of approaches to diagnosis, modes of treatment and prevention, and the use of Aquaflor to reduce mortality in intensively reared HSB that are naturally infected with S. iniae. Much of the information presented here reflects experience of the senior author (Vaughn Ostland), which includes nearly 10 years in health management of intensively reared HSB in a semi-recirculation culture system located in the low-desert region of southern California (Coachella Valley).
Streptococcus iniae as an aquaculture pathogen
Streptococcus iniae, a gram-positive, coccus-shaped bacterium, was initially isolated about 30 years ago from a captive Amazon River dolphin (Inia geoffrensis). Since then, S. iniae has become an adept pathogen in many freshwater and marine fish species, and its association with cellulitis and osteomyelitis in humans often follows the handling of whole, fresh fish. Based on personal experience at a commercial facility with significant levels of endemic S. iniae in HSB and tilapia, as well as in vitro and in vivo experimentation with this pathogen, it is our opinion that S. iniae is primarily an aquatic animal pathogen, although measures must be practiced to minimise exposure and unnecessary risk to farm workers and fish.
Streptococcus iniae appears to have a global distribution since there are over 30 freshwater and saltwater fish species reportedly susceptible to the disease. Both wild and economically important cultured fish species can be affected.
Annual economic losses due to S. iniae, which can result in significant mortality, are estimated to be in the hundreds of millions of dollars. Given the ubiquity of this pathogen, it is likely that S. iniae can infect just about any fish species propagated under intensive aquaculture conditions.
Epidemiology of S. iniae in naïve hybrid striped bass
The seasonal prevalence of S. iniae infections in naïve HSB reared in the Coachella Valley appears to be related to water temperature. During late winter months when tank water temperatures were less than 20° C (68° F), overt clinical outbreaks were not seen. As tank water temperature increased to the range of 21° C (69.8° F) to 24° C (75.22° F), the prevalence of clinical disease, based on clinical signs and gross anatomical changes, could increase quickly to 20 per cent to 50 per cent of tanks. In hot summer months when water temperatures were above 25° C (77° F), the prevalence of S. iniae infections would commonly approach 60 per cent.
S. iniae is spread throughout the aquatic environment via horizontal transmission, but the exact mechanism of transmission is unknown. The hypothesis is that entry into the host may be oral or across epithelial surfaces such as the skin, gills, nares or intestinal tract. Many warmwater finfish, including HSB, are cannibalistic, a behavior that probably contributes to the rapid horizontal spread of S. iniae. Indeed, some experimental co-habitation infection models rely on this behavioral trait to transmit the disease. Furthermore, laboratory infection models have been developed to routinely transmit S. iniae infection to naïve HSB and tilapia by immersion, which implicates water-borne exposure as a common method of transmission in cultured fish.
Wild fish can also harbor S. iniae and should be considered potential carriers. Wild fish kills associated with S. iniae have been reported, underscoring the role of carrier fish in the dissemination of S. iniae throughout aquaculture.
Clinical signs of S. iniae infection

Morbidity is one of the earliest behavioral indicators of S. iniae infection in cultured HSB. The most common clinical sign is lethargy; the greatest number of moribund animals are found at or near the water surface near the outer tank edges, where water flow is reduced. Fish infected with S. iniae consistently display dark skin pigmentation and labored breathing, although neither of these signs is specific for S. iniae infection in HSB. As the infection progresses, individual fish will be observed “spinning” through the water column.
Fish in the late stages of S. iniae infection can be observed trying to leap out of the tank. Soon after, mortality can increase rapidly by several-fold over the course of a few days.
An interesting behavioral characteristic seen in S. iniae-infected HSB is a continued interest in feed throughout the early stages of infection. This has significant implications, since it can signal the need for oral antibiotic treatment early in the course of the disease, which increases the likelihood of a better outcome.
Once morbidity is established in the production unit, mortality rapidly progresses.While many factors can contribute to cumulative mortality, fish age and water temperature seem to be the most common for naïve HSB reared in circular, concrete tanks in semi-recirculation conditions in southern California.
Young fish are most susceptible with rapid onset of mortality occurring in a geometric fashion over the course of 2 to 4 days when the water temperature is above 25° C (77° F). Older fish appear to have greater resistance, possibly due to better innate or adaptive immune function. If left untreated, overall mortality due to S. iniae infection can approach 30 per cent to 60 per cent and can be even greater and occur more rapidly if other factors are present, such as a change in husbandry practices just before the clinical outbreak.
Diagnosis of S. iniae infections in hybrid striped bass
Gross external signs of an S. iniae infection in HSB include not only dark skin pigmentation but ocular opacity and bilateral exophthalmia (pop-eye) as well as the presence of small, rounded, swollen, raised regions at the base of the caudal peduncle, which are known as “caudal pustules”(Figure 1).
Internally, common findings are a swollen head kidney and spleen and mild inflammation of the heart surface (epicarditis). Any or all of these signs can be seen in any one animal; however, the severity of gross lesions is often a function of fish age, the length of time fish have been infected and the water temperature. Cardiac changes seem to be more common in older HSB that have been infected, treated and survived an S. iniae infection.
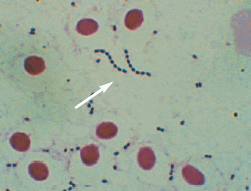
A quick, easy method for the presumptive diagnosis of S. iniae infections in HSB can be made with a gram stain of tissue imprints or blood smears prepared from infected tissues (spleen, head kidney, brain, blood). This can be performed during necropsy using commercially available staining reagents and supplies. The presence of gram-positive cocci in infected tissues will help confirm that Streptococcus sp. is present in the infected tissue (Figure 2).
The “gold standard” for confirming the presence of S. iniae in infected HSB is to perform a bacteriological culture of affected organs.We routinely culture the brain, followed by the head kidney, because S. iniae is easily recovered from these organs during the early phase of infection. Using standard aseptic methods to collect infected tissue, the samples should be streaked over the surface of a blood agar plate, placed in an environment designed to reduce desiccation of the agar surface during incubation and held at an appropriate temperature suitable for recovery—usually 25° C (77° F) to 30° C (86° F).
Under these conditions, small colonies are visible by 24 hours post-inoculation with evidence of strong-alpha to weak-beta hemolysis. By 48 to 72 hours post inoculation, gram-positive cocci (in pairs and short chains) produce individual whiteto tan-colored, raised, circular colonies that display distinct beta-hemolysis. An individual colony is removed and propagated on a new agar plate to confirm purity and begin preliminary bacterial identification and antibiotic sensitivity testing. If adequate diagnostic facilities are unavailable, moribund animals must be transported to a local veterinarian or regional diagnostic laboratory experienced in aquatic pathogen culture and sensitivity.
Prior to administering medicated feed, the clinical isolate recovered from an affected fish should be presumptively identified as S. iniae and shown sensitive to florfenicol, the active ingredient in Aquaflor. Confirmation of the identification can be performed at a later time and usually involves traditional phenotypic characterisation with molecular methods such as direct 16S rRNA sequencing or probing genomic DNA with S. iniae-specific PCR primers to confirm the most closely related species of the genus Streptococcus.
Polyclonal or monoclonal S. iniae-specific antiserum is useful in a diagnostic aquaculture laboratory for rapid bacterial identification. For example, on a glass slide, commercially available S. iniae-specific polyclonal antiserum can be mixed with a suspension of streptococcal cells prepared from the agar plate and observed for visible clumping. This simple agglutination assay is a serological approach to help confirm bacterial identification based on phenotypic characterisation. Since cross-reactivity can be an inherent problem in agglutination assays, the specificity of the antiserum must be established using known species of Streptococcus before results can be interpreted with confidence.
Histopathology can also be an effective tool for managing and diagnosing S. iniae infections in HSB. The presence of small clusters of cocci that are morphologically similar to streptococci and associated with characteristic lesions in the heart and meninges of the brain are histopathological criteria necessary to help confirm streptococcal septicemia (Figure 3).

One drawback to histopathology is the length of time it can take to receive an interpretation and report; this can be offset by the fact that it is sometimes possible to determine whether multiple pathogens are involved in the disease outbreak.
Successful treatment of mixed infections, which are not uncommon in intensive HSB culture, can be problematic and may depend on whether the secondary pathogens are bacterial and, if so, whether they are sensitive to the antibiotic. These pathogens can be mucosal, systemic or sometimes both.
In our experience, the presence of parasitic mucosal pathogens was merely a reflection of the systemic health status of the fish. The most common protozoan parasites seen on the skin and gills of S. iniae-infected HSB include members of the genera Trichodina, Ambiphyra, Epistylus and Ichthyobodo.
Similarly, mixed systemic bacterial infections have also been diagnosed; the most common encountered include unique strains of Mycobacterium marinum and an emergent aquatic Francisella sp. We were never able to ascertain whether S. iniae rendered HSB more susceptible to infection by these gram-positive and gram-negative intracellular pathogens, respectively. Treatment with Aquaflor was more successful in HSB without these other pathogens.
Determining antibiotic sensitivity
If antibiotic sensitivity is evident in vitro, there is a chance it will be effective in vivo. Perhaps the most common method used to determine bacterial sensitivity to an antibiotic is the standard agar-disk diffusion.
From a practical perspective, it is important to quickly establish antimicrobial sensitivity so treatment of the affected tank can begin readily. This will dramatically improve treatment success, reduce overall mortality and lost production potential as well as minimise unnecessary pain and suffering.
Based on information provided in the product insert from the antimicrobial sensitivity test disk manufacturer, a clinical isolate of S. iniae was considered sensitive to Aquaflor if the diameter of the zone of inhibition for the florfenicol disk (30 ug disk potency) was ¡Ý21 mm. This interpretive criterion was based on the disk diffusion zone diameter data reported for betahemolytic streptococci grown in the presence of chloramphenicol. Typically, laboratories use cattle zones of inhibition and a zone of inhibition >19 mm indicates that an organism is sensitive to florfenicol. Historical collection of florfenicol zone-ofinhibition data from clinical S. iniae isolates recovered from HSB reared in southern California revealed that the mean diameter of the zone of inhibition was 27 mm (n=271 isolates, range from 22-31 mm). This provides evidence that S. iniae has not developed resistance to Aquaflor.
Oral treatment of S. iniae infections with Aquaflor
The most effective way to treat HSB infected with S. iniae is to deliver the antibiotic orally in feed at the recommended dosage of 10 mg active ingredient per kg of fish per day for 10 consecutive days. Our standard ration for HSB was a commercially available trout production diet (Silver Cup, Murray, Utah), with the protein-to-fat ration adjusted to reflect fish age and the phase of production.
Aquaflor can be included in feed either by surface coating pellets with the drug suspended in an aqueous carrier or by incorporating Aquaflor inside pellets during manufacture. During summer months, it was not uncommon to simultaneously treat multiple production tanks of S. iniae-infected HSB, which made it more convenient to have the antibiotic incorporated into pellets at an inclusion rate of 4 pounds of Aquaflor per ton of feed and fed at 1 per cent biomass to deliver 10 mg of florfenicol/kg of fish.
We administered feed medicated with Aquaflor as the sole ration for the entire 10-day treatment period. Since this drug was used under the direction of a US Fish andWildlife Service Investigational New Animal Drug (INAD) exemption, a 28-day withdrawal period was established before fish could be harvested for human consumption.
Normal, baseline or expected mortality for any aquaculture facility should be established so a rational decision can be made about when to begin oral antibiotic treatment. For example, with intensively reared HSB in the Coachella Valley, total mortality by tank was generated on a daily basis to establish the normal baseline mortality at one-half of 1 per cent of the tank population per 28-day period. Baseline mortality will vary among aquaculture facilities, but these data are necessary to establish and maintain daily operating procedures and treatment decisions consistently. Therefore, determining when to initiate feed medicated with Aquaflor merely involved establishing the “entrance criteria,” which was a calculation based on the increase in daily mortality for 3 consecutive days (over normal or baseline mortality) by treatment group (the tank) and confirming the presence of S. iniae in the treatment group via routine culture of moribund fish from tanks with clinical signs of S. iniae infections. Also of importance is that the representative S. iniae isolate from that treatment group was sensitive to Aquaflor in vitro.
Another entrance criterion we used to help quickly initiate treatment of HSB with Aquaflor and minimise mortality was to perform routine fish health examinations of moribund HSB from tanks with a dramatic increase in morbidity. In addition, a positive culture of S. iniae-like colonies was required from at least one infected fish before treatment was considered.
Based on laboratory and field experience, we did not delay treatment until sensitivity to Aquaflor was confirmed, since HSB are extremely sensitive to S. iniae infection and mortality can escalate rapidly. Antimicrobial sensitivity testing was generally completed within the first couple days after treatment began. This approach helped reduce overall mortality, especially during warm months.
Field experience with Aquaflor
Field experience has demonstrated that feed medicated with Aquaflor has an excellent safety profile. It also has excellent palatability in all age classes of HSB ranging in size from 2 or 3 g to 1 kg. As described previously, infected HSB display an aggressive feed response early on during an S. iniae outbreak, which ensures that most fish in affected tanks will consume the medicated feed, increasing the likelihood of treatment success.
Accurate delivery of medicated feed to tanks of infected HSB required the calculation of total tank biomass. This required the total number of fish in the tank and the average weight of each fish to be known so that the quantity of medicated feed needed to treat the tank can be calculated accurately.
Since tank biomass was inventoried only once per accounting period (i.e., every 28 days), there were often inadequate biomass data available at initiation of treatment because fish had grown since the previous inventory. Consequently, we estimated total biomass and the amount of medicated feed to deliver. Since Aquaflor has a wide margin of safety, we erred on the high side and delivered slightly greater quantities of the antibiotic to tanks of infected fish, which received their daily ration of medicated feed during three hand feedings.
Standard protocol for delivery of feed medicated with Aquaflor to infected HSB would be to round off the weight of medicated feed required for one particular tank to the nearest half bag (i.e., nearest 25 pounds of feed) and provide this as the sole ration for the recommended treatment duration of 10 days.
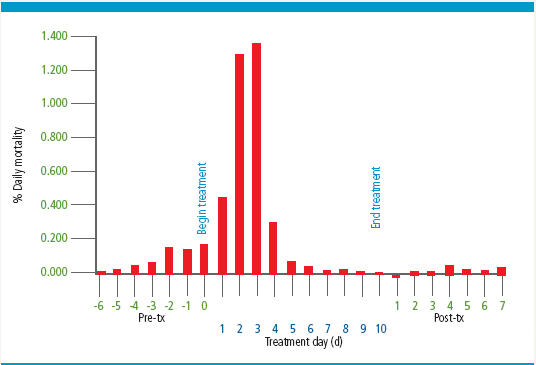
Monitoring long-term performance of HSB following oral administration of Aquaflor showed that treatment did not contribute to any obvious reduction in growth or feed conversion. In some instances, growth was reduced immediately after completion of treatment but this was almost always shortlived. The transient reduction in growth was more pronounced in fingerling HSB with an average weight less than 10 g that had recently received an intraperitoneal vaccination with an oil-adjuvanted autogenous S. iniae biologic. Similar to that reported for salmonids or catfish, a dramatic reduction in mortality was common roughly 3 to 4 days after S. iniae-infected HSB received Aquaflor treatment (Figure 4).
Sometimes, reduced mortality did not occur until 5 to 7 days after treatment started and the reason was not always apparent. In some cases, a secondary bacterial pathogen that was not sensitive to florfenicol was recovered; in other cases, a concomitant parasitic gill or skin infection was found, especially in older fish reared under recirculation conditions.
Other possibilities for a slow response to treatment included the length of time to obtain culture and sensitivity, the age of fish to be treated, the number of times the tank had received previous medicated feed treatment or perhaps vaccination status. In these instances, mortality would be slow to return to the original entry level prior to treatment until several days after the 10-day treatment ended. In an intensive aquaculture facility that is fighting multiple pathogens, mortality during the post-treatment period is common. The results we obtained with Aquaflor in this setting are a testament to its efficacy.
Prevention of S. iniae infections
Health management goals for aquaculture must include disease prevention and efforts to minimise the adverse effects of disease outbreaks that occur. Maintaining healthy rearing conditions, providing proper nutrition, routine fish health monitoring and the use of vaccines can collectively help prevent disease outbreaks. However, when a disease outbreak occurs, timely treatment with safe, effective therapeutic drugs such as Aquaflor can reduce explosive mortality.
Vaccination has the potential to reduce streptococcal mortality in aquaculture.18 However, to date, there is no commercially available vaccine in the US to prevent S. iniae-associated mortality in HSB. Ostland has considerable experience in the development of an autogenous injectable S. iniae vaccine in HSB and has conducted field trials demonstrating vaccine efficacy under intensive production conditions at a commercial facility. HSB that were immunised with the S. iniae vaccine generally experienced lower mortality than unvaccinated HSB. Collectively, our experience suggests that Aquaflor can dramatically reduce S. iniaerelated mortality in HSB previously vaccinated for this pathogen.
Conclusion
S. iniae is a common bacterial pathogen of intensively reared HSB in southern California.While relatively easy to diagnose, consistently successful treatment outcomes can be more difficult to achieve.
Our experience has shown that Aquaflor can significantly reduce mortality in HSB that were naturally infected with S. iniae. Furthermore, when administered as directed, Aquaflor was found to be both effective, safe to use and did not have a negative impact on the commercial production of HSB.
Treatment success depended on how quickly Aquaflor was administered to sick fish. Fast initiation of treatment required rapid diagnosis and confirmation that the S. iniae isolate was sensitive to florfenicol.
The judicious use of Aquaflor in HSB for over 5 years did not appear to induce antimicrobial resistance in strains of S. iniae recovered from moribund HSB at this facility. All in all, Aquaflor is an important fish health management tool that can quickly and effectively reduce S. iniae-associated mortality in HSB.


