
It is not necessary to attach the canula by stitching, since the pressure in the blood vessels pushes the tube against the bone of the nose. The tube is thin and floats on the surface of the water in between blood sampling times. The fish feeds again a couple of hours after the canula has been inserted, and within 24 to 48 hours it is back to normal and indistinguishable from other fish.
Need for frequent sampling
It is necessary to take frequent blood samples from fish in order to find out how well it tolerates and utilises new feed ingredients. In the absence of good methods for blood sampling, this has proved to be somewhat tricky. Now, however, the fish can swim normally in a tank and have blood samples taken almost without noticing what is going on.
The method
The method is called cannulation, and has been practiced in laboratory research since 1994. It is only recently that it has become good enough to be used on a larger scale. The method is now on its way in as a standard method in production environments and the industry is very interested.
PhD student Ms Brankica Djordjevic has in her doctoral work among other things worked on optimizing, refining, compiling and testing the method, which has been tried out on salmon and rainbow trout. It has been tested on new feed sources in order to prove that the technique is up to the mark.

The need for new feed sources for fish
Fish eats fish, but there is a lack of fish meal supply in the world, and the aquaculture industry will need new feed sources in the near future.
Must know more about the fish
It is necessary to get to know the fish better – because of the feed situation, because consumers demand better welfare for the fish, and because it makes good financial sense. A fish that leads a good life is less frequently ill, eats, grows and utilises the feed better than a fish that does not have optimal conditions.
Cannulation is cost-saving
Consumers want red salmon meat. Up until now it has been necessary to feed the fish on test feeds containing pigments, wait six months, slaughter the fish and check if the meat is red. Besides taking a long time, this has shifted 40 000 fish. Seeing that pigment content also shows up in the blood and can indicate what muscle colour the fish will have six months later, we can now have the answer after two weeks and 16 fish. The method can profitably be used as an indicator of feed ingredients that merit further use in a larger scale production experiment.
New discovery on nutrient uptake
During the search for new food for fish, the method has also been used on feeds containing soy, peas and fish meal. From blood sampling the researchers found something they had never seen before. It was known that soy and peas in fish feed led to reduced growth, but the reasons for this were not clear.
The blood samples showed varying uptake profiles for the different feed types. Protein showed a steady uptake over time when using feeds containing fish meal, while especially when soy was involved, protein levels rose and fell very rapidly. Fish do not store amino acids. They must be taken in via the food. The results show that as far as protein goes, all amino acids must be present there and then in order for metabolism and growth to occur. On fish meal, the fish seems to grow 24 hours of the day, while when using soy, the fish grows only intermittently.
A natural habitat is a prerequisite
To get correct results from the blood samples it is vital that the fish behaves normally, swims freely and eats without being affected by the technique itself.
The problem up until now has been that is has been impossible to examine the fish often enough and in such a way that it at all times leads a normal life and that the physiological reactions within the fish continue undisturbed.

One fish to the tank
The fish is alone in the tank, and it has been challenging to create an environment where salmon can thrive on its own. In order to make it homey, currents are created in the water, and the fish has a place to hide. The fact that the fish does not have to compete with others for food is surely one cause of feed uptake being somewhat lower than what is usual in ordinary fish facilities, but there is no indication that the fish eats less than it feels like.
I have spent a lot of time checking that the fish lives in a natural environment and that it behaves naturally, says Ms Djordjevic .
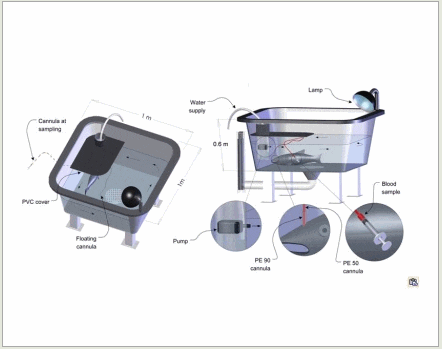
The light from the lamp attached to the edge of the tank creates a shadowy hideout underneath the PVC cover, where the fish stays quite happily in the stream from the water pump.
Blood sample from the abdomen
The blood goes from the intestine to the liver. It is therefore of interest to get blood samples just before the liver in order to see what is absorbed and metabolised in the liver. A method where a catheter is inserted between the intestine and the liver has also been tested during the doctoral work, in cooperation with researchers at University of British Columbia (UBC), Canada.
So far the work on this method is half way completed. As opposed to cannulation through the snout, this method involves surgery. There are still some difficulties to be overcome concerning the operation technique, to ensure that the fish recovers and starts feeding as usual quickly after the procedure.
Anaesthesia – another challenge
The anaesthesia technique also needs to be improved, as today’s method is rather annoying to a fish. The longer a fish remains under anaesthesia, the more stressed it becomes.

How often can blood samples be taken?
Ms Djordjevic has also looked into the question of how frequently and for how long a time period it is possible to take blood samples. Within reasonable limits, the fish seems to cope very well. Four samples in 24 hours, two days a week for eight weeks seems to work well without affecting the fish. After that, the canula getting clogged up poses the problem.
Testing dietary supplements
Giving a supplement in the form of the fungus Lentinula edodes has proved to have a positive effect on the immune system in humans. While giving this supplement to fish, Ms Djordjevic looked to see whether this improved the immune response in fish exposed to a pathogenic agent (lps – lipopolysaccharide, which mimics bacterial infection) – which it turned out to do. The genomic tools used in this experiment are relatively new in connection with fish. They allow measuring the expression levels of gene activity.
By slaughtering the fish before it fell ill, we examined the changes in the genes that express the immune response. Fish that were fed with the supplement had a far milder reaction than fish in the control group, which did not get the supplement. A pronounced immune response over a long period of time can lead to illness and death because it takes a lot of energy out of the fish, says Ms Djordjevic.
In an ordinary experiment to test new medication or other products, large groups of fish are exposed to pathogens, and one follows the group to observe how many fall ill and die. Using the lps model of inflammation in combination with genomic tools, the number of fish needed is far lower, and the fish does not get to fall ill before it is slaughtered.
Combining techniques
In the future, Ms Djordjevic hopes and believes that it will be possible to combine genomic tools with cannulated fish.
The plan is to try out these techniques to evaluate new feed sources and different stress reactions in fish, Ms Djordjevic concludes.
Ms Brankica Djordjevic is 33 years old and originally comes from Serbia. She has a degree as Agricultural engineer for Animal Sciences at Belgrade University. She also has a master degree in aquaculture at UMB.
The trail lecture and public defence took place on 5 December 2009 at IHA. Title of the thesis: Physiological and genomic responses in salmonids with special emphasis on welfare and novel feed ingredients. Prescribed Subject of the trail lecture: Immune suppression after stress
Assistant supervisors have been Professor Magny Thomassen, IHA, Professor Olav Rosseland, INA and researcher Øyvind Øverli, IHA.
March 2010

