How widely used are lumpfish in the salmon industry?
The use of cleaner fish for sea lice control has grown exponentially in the last years, and is expected to continue doing so, with an estimated 26 million stocked in Norway alone in 2015 and an estimated 50 million, predominantly hatchery reared lumpfish, being needed in 2020 according to Powell. The development of non-medicinal lice control strategies, which include the commercial production of cleanerfish, has been singled out as the area in most urgent need of research and is a major priority for the industry and governments.
As in other European salmon farming countries, sea lice are one of the most significant challenges facing the salmon industry in Ireland and cleaner fish are widely used. They are considered a very promising approach to sea lice control, especially as the majority of salmon production in Ireland is organic and the number of treatments is restricted by EU regulation. The Irish industry largely relies on the use of wild-caught wrasse, with over 600,000 being stocked since 2015. Hatchery reared lumpfish use to date has been lower, around 300,000 have been deployed in salmon sea pens since 2015, but the long-term aim is to increase the use of hatchery reared, vaccinated lumpfish of known disease status.

What’s the main significance of your discovery of Tetramicra brevifilum in lumpfish?
High cleanerfish mortality due to infectious diseases has been a big problem in all countries using cleanerfish and we know very little about infectious diseases of lumpfish. The most important part of this discovery is describing T. brevifilum as a significant pathogen in lumpfish and publishing the symptoms of the disease and details of the outbreak. T. brevifilum is a commercially significant pathogen of turbot (Scophthalmus maximus) and has also been isolated from black anglerfish (Lophius budegassa), proving that it is not host specific. It was not previously known that lumpfish could be infected. In Ireland, T. brevifilum caused severe clinical disease and significant mortality in broodstock. Some microsporidian species are common incidental findings with no known clinical impact on the fish, so it is important to know that T. brevifilum is not in this category but is potentially a serious pathogen. The clinical symptoms, histopathology and the appearance of the microsporidian spores described in the publication are quite characteristic and should enable others working with lumpfish to recognise the disease.
Do you think the infection developed in the wild or after the fish were placed in captivity?
Microsporidia can be present in the host organism for a long time without causing clinical disease, so it is possible they were infected before capture. This is often the case in mammals, where clinical microsporidiosis is often linked to an impaired immune system. This has also been suggested as being the case in Tetramicra outbreaks in turbot. In Ireland, the disease has been seen in lumpfish held in two separate facilities but originating from the same wild-caught batch of fish, which is a possible indication that the microsporidia were present in wild lumpfish. Since then the disease has been seen in isolated cases in lumpfish mortalities from sea pens, though bacterial diseases and AGD were also present and were considered the main causes of mortality. In one case the infection was seen on a 0.2g juvenile lumpfish hatched in captivity with no connection to any previous cases, which means there was either vertical transmission or the fish was infected in captivity. T brevifilum was first described in wild turbot populations in southern England and it must be assumed that it is present in wild fish around Europe.

© Fish Vet Group
Is there a way of developing a screening process to ensure the pathogen is not brought in to aquaculture facilities by wild-caught lumpfish?
My earlier research led to the development of a PCR protocol for the detection of T. brevifilum, so it is possible to screen lumpfish for the infection. Based on the cases seen, the liver seems consistently heavily infected and would be a good organ to sample, though in clinical cases most organs are affected. We also detected T. brevifilum in the blood of infected fish, so lethal sampling is not necessary. Wild-caught lumpfish broodstock are often killed after spawning/stripping and samples are taken from different organs to test for pathogens of concern. The eggs are kept separately until results are available and can be discarded if any significant infections are detected. Tissue samples for T. brevifilum could be included in this protocol, but testing the fish through a blood sample before spawning would be a significant advantage. We still need to evaluate how reliably the PCR picks up on subclinical infections, but it should be possible to develop effective screening protocols.
Have symptoms similar to those in the fish you examined been identified before?
Some of the symptoms - like protruding eyes, fluid in the abdominal cavity or lethargic behaviour - are very unspecific and are seen in a variety of infections. White flocculent material in the eyes has been described for Myxobolus albi infections, and Kudoa islandica is a differential diagnosis for white nodules in the muscle. However, externally visible “blisters” with milky content, which are also seen on internal organs, make the clinical presentation of Tetramicra quite unique. On histology, the xenomas can show characteristic features like a microvillous surface, and the spores themselves show a distinct posterior vacuole with an inclusion body when viewed on wet mounts. This has only been described in one other fish infecting microsporidian (Nosemoides sp.). So, all in all, the clinical disease is quite distinct and a diagnosis is often possible after clinical examination, a wet mount and histology.
What impact does T. brevifilum have on the lumpfish and how infectious is it?
The impact in clinical cases was very high. All internal organs were affected and a large proportion of the more affected organs, such as the liver, was replaced by cysts containing microsporidian xenomas. It is remarkable that lumpfish kept feeding and showed no obvious behavioural symptoms for as long as they did, though behavioural indicators are generally very unreliable in lumpfish. Our data to date is limited to severe clinical cases and occasional incidental findings which may have turned into clinical cases if left to progress. There are reportedly indications that turbot can overcome the infection and we do not know if this may be the case with lumpfish as well. There is not enough data to say how infectious the disease is at present and we do not know how T. brevifilum is transmitted. The release of infectious spores has been described as dependant on the death of the host, though the very superficial location of T. brevifilum cysts makes it seem likely that spores will be released before death or very shortly afterwards and horizontal transmission is considered likely. It is important to know that microsporidian spores can retain their viability in water for over a year at 4°C so the potential for re-occurring infections after spores have been released into an area is considered high.
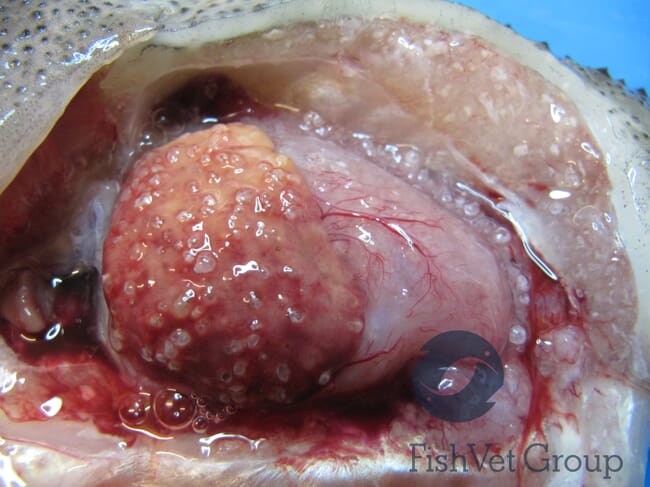
Do you think there is scope to develop treatments against T. brevifilum?
There is no treatment licensed for microsporidiosis in fish at present, though treatments, for example with albendazole, have been used successfully in some other species such as honey bees. However, microsporidiosis can be very hard to treat - depending on species, site and severity of infection. As infections in lumpfish are often only detected at an advanced state, a successful treatment will be difficult. Good hygiene, husbandry and fish welfare are likely essential in preventing infection and disease.
What other diseases have been found in lumpfish to date?
A wide variety of bacteria and parasites as well as some fungi and viruses have been found in lumpfish to date. Bacteria have so far been the most problematic in terms of mortality and Pseudomonas anguilliseptica, Vibrio anguillarum, Vibrio ordalii, Tenacibaculum spp., Pasteurella sp., Piscirickettsia salmonis, atypical Aeromonas salmonicida subspecies and Moritella viscosa are examples of species that have caused mortality in lumpfish. Amoebic gill disease (Neoparamoeba perurans infection) has caused high mortality in lumpfish both at sea and in land-based facilities. N. perurans is also the only pathogen that has been proven to be transmissible between lumpfish and salmon to date.
Caligus sp., scuticocilliates, Gyrodactylus sp., Trichodina sp., Kudoa islandica and Myxobolus albi are other examples of parasites that have been problematic in lumpfish. Known fungal pathogens of lumpfish include Nucleospora cyclopteri (microsporidia) and Exophiala spp.. Two viruses have been described in lumpfish to date, a ranavirus of yet unknown significance and a flavivirus to which significant mortality has been attributed in Norway. New diseases are being described regularly in lumpfish, though diagnosis of outbreaks in sea pens is often difficult and it must be assumed that many disease outbreaks are going undiagnosed.
Do lumpfish infected with these diseases pose a risk to the health of salmon or purely to each other?
The risk posed to salmon is unknown. T. brevifilum is present in the marine environment and has never been described in salmon or a related species so the risk is considered low. However, the infection has so far been limited to benthic species, possibly due to infection through a benthic vector. Salmon in net pens may not have come in contact with T. brevifilum on a significant scale before, which may change through co-habitation with infected lumpfish.
Does the discovery strengthen the argument to close the lumpfish rearing cycle?
Yes, T. brevifilum is among the pathogens that could potentially be avoided by closing the lumpfish rearing cycle. The risk of true vertical transmission is unknown, but vertical transmission has been demonstrated for several microsporidian species. The high numbers of spores in all organs including the gonads and the potential for spores to survive in the environment means that infection or contamination of the eggs and subsequent infection of larvae is also considered possible.
What are the main areas of research that the FVG are likely to be concentrating on in the wake of the discovery?
New diseases are regularly appearing in lumpfish and the research focus of FVG has shifted away from T. brevifilum to other pathogens previously unknown in cleanerfish, such as Piscirickettsia salmonis in lumpfish, piscine myocarditis virus in wrasse and the newly identified ranavirus in lumpfish. However, as cases of Tetramicra brevifilum have been diagnosed during routine visits and will likely reappear in the future, this species will likely return into the focus of research. Future research needs include optimizing a screening protocol, investigating possible treatments and modes of transmission, and comparing isolates from different origins.



